Lightning-on-Demand Could Create the Green Fertilizer We've Been Waiting For
Here's how to replace the Haber-Bosch process with something more decentralized
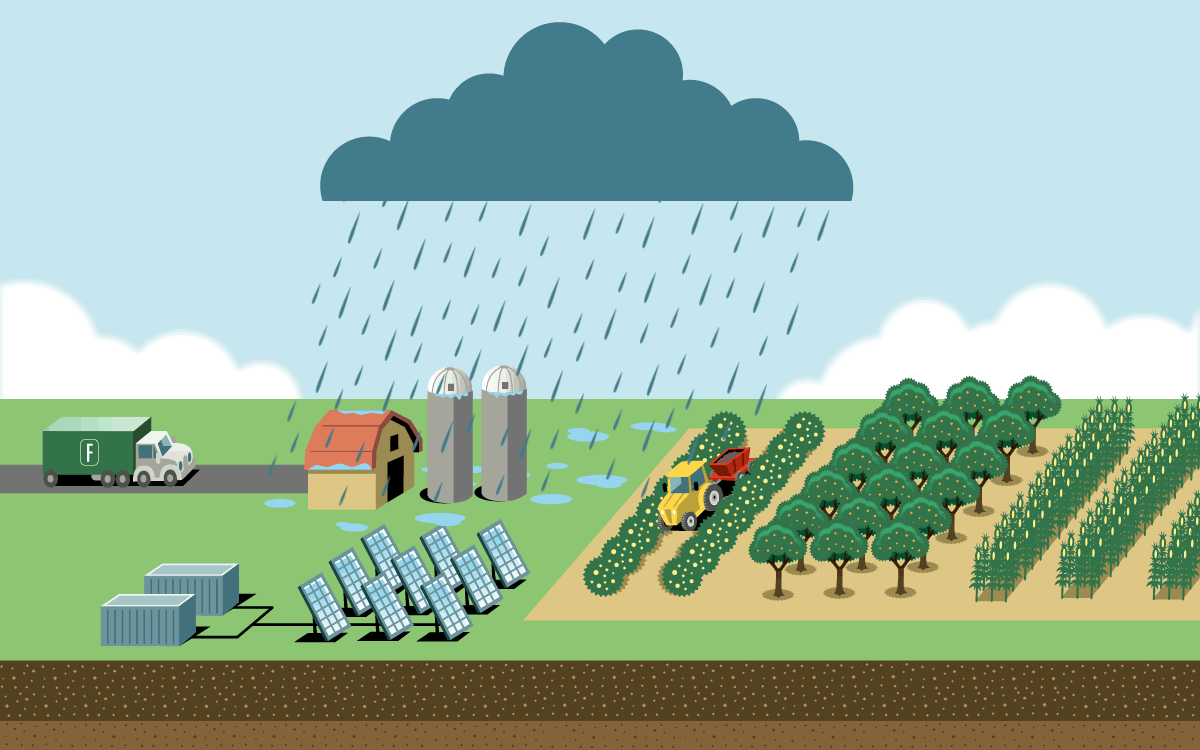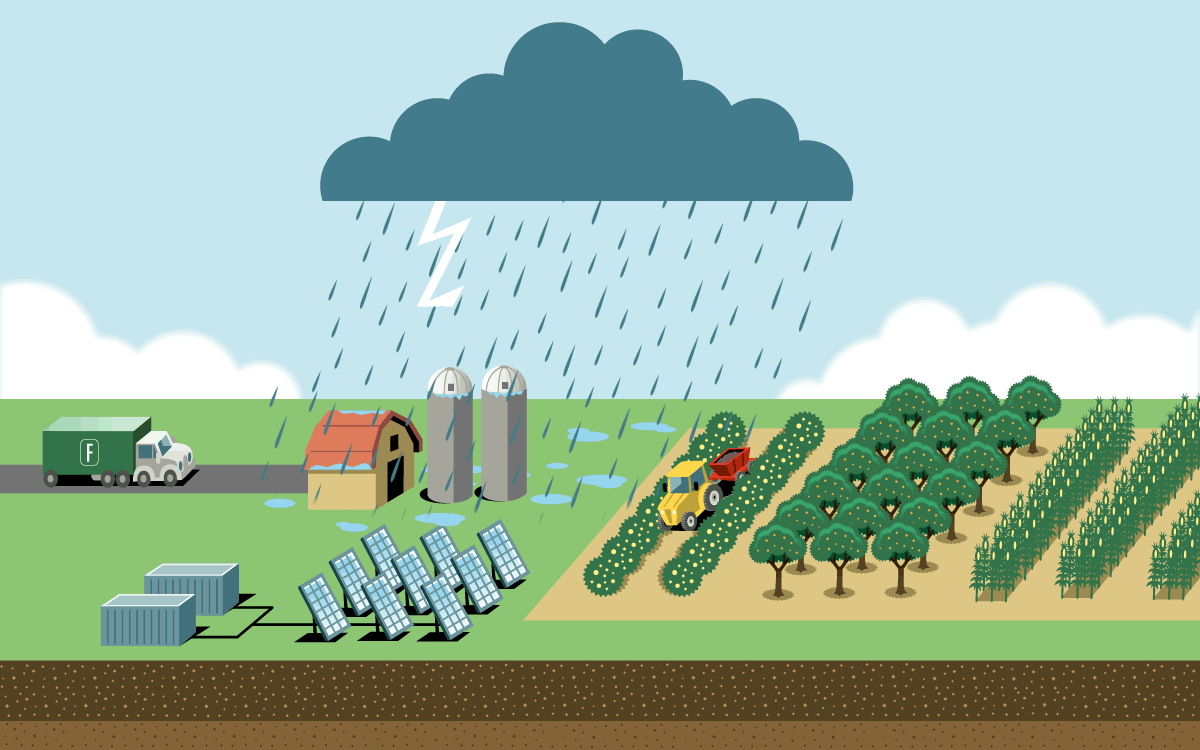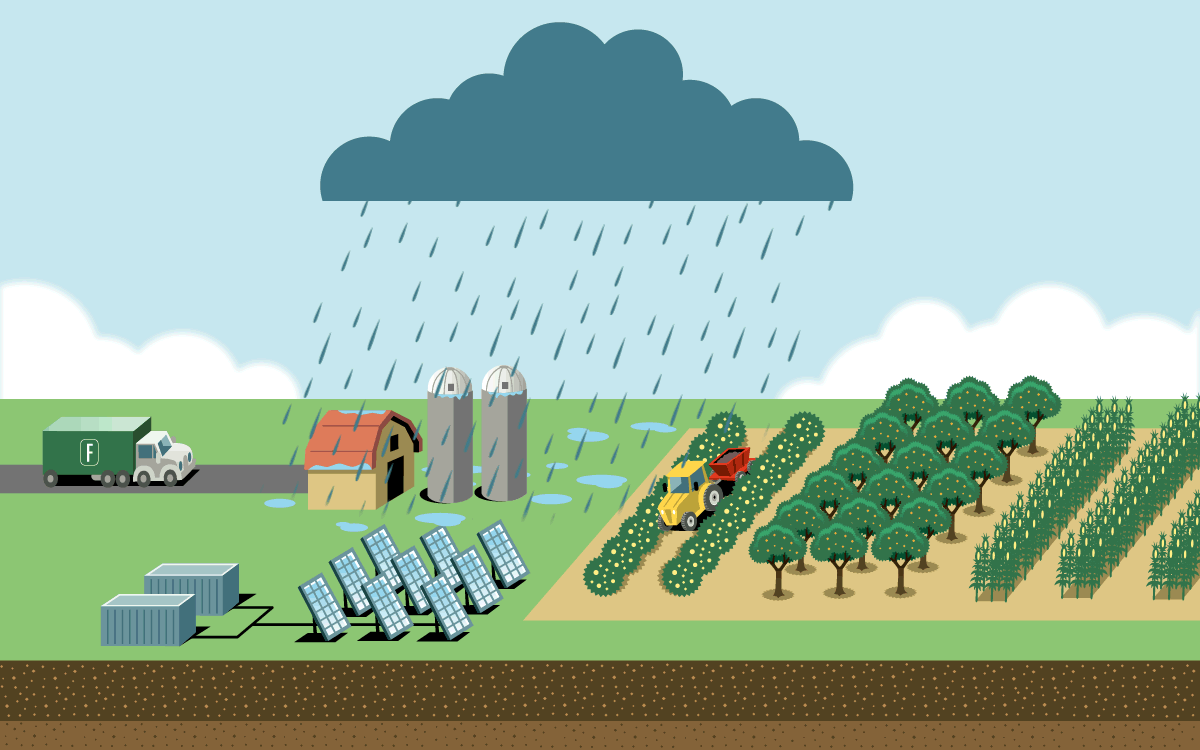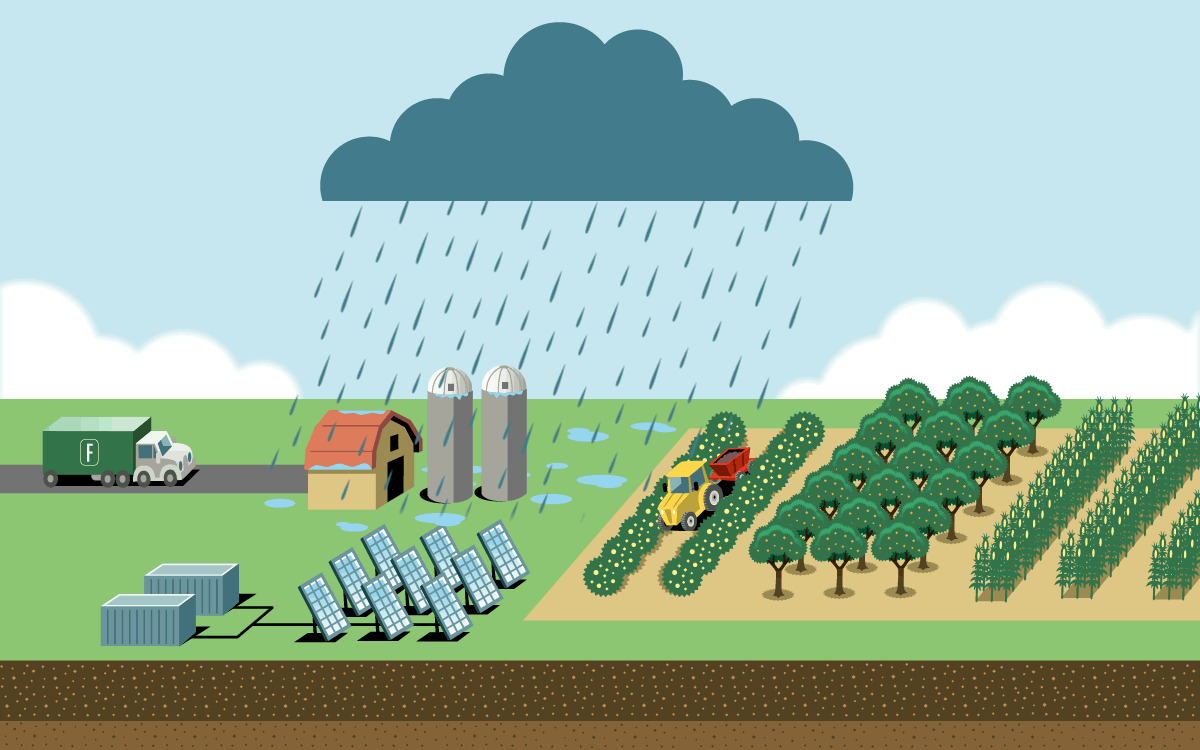
To feed a global human population of 8 billion and counting, we need more nitrogen than is naturally available in soil. Industrial agriculture relies on the Haber-Bosch process, which uses high pressure and temperatures to create nitrogen fertilizer in the form of ammonia. But the process is responsible for as much as 3 percent of global emissions. Most result from the field application of ammonia fertilizer, which releases nitrous oxide, a greenhouse gas even more potent than carbon dioxide. The rest are from fertilizer’s energy-intensive production and its transportation.
To reduce these emissions, some companies and research groups are working to make “green ammonia”—powering the Haber-Bosch process with renewable energy. But others are focused on a completely different way of fueling plants: lightning on demand.
HOW IT ALMOST WORKED
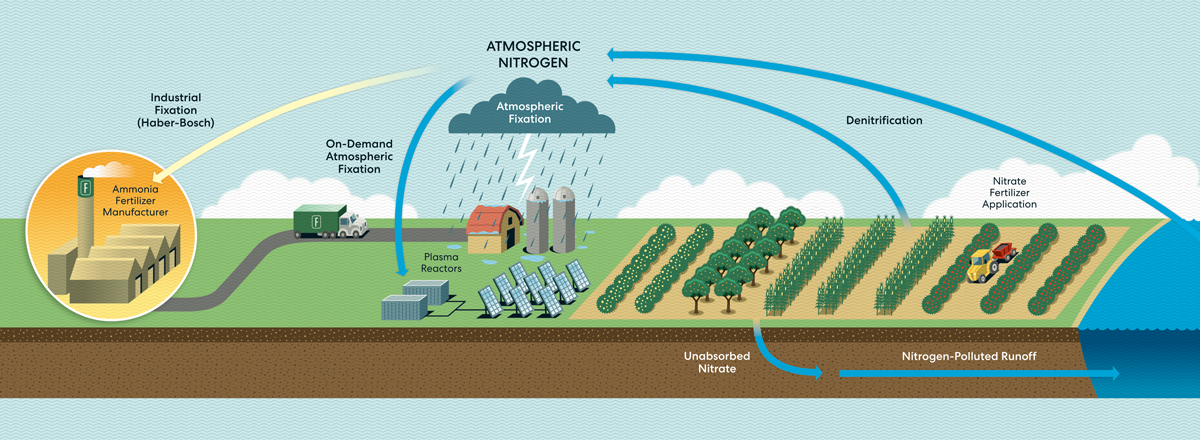
Nitrogen naturally occurs on Earth, moving in a cycle from the atmosphere to the surface and back (the latter step is called denitrification). Atmospheric nitrogen is too tightly bonded for plants to absorb it directly. This essential nutrient must be broken down and recombined with other elements (or “fixed”) to form the ammonia or nitrate plants can use.
While most fixing is done by bacteria, lightning is powerful enough to split super-stable nitrogen molecules in a process called atmospheric fixation. People first used electricity to create nitrogen-derived fertilizer in 1903, but Haber-Bosch, invented soon after, proved more cost-efficient and took off. Now electricity-based methods are catching up.
HOW IT COULD WORK TODAY
A plasma reactor can create energy bursts similar to lightning using just air, water, and electricity. The reactor produces nitrogen dioxide, which dissolves in water to form nitrate. When nitrate is diluted further, farmers can apply it directly to crops through spraying or irrigation lines. It can also be dehydrated into granules or mixed with lime to become calcium nitrate, a popular premium fertilizer.
The technology is small enough to fit inside a solar-powered shipping container on a farm, which would eliminate the emissions from the fertilizer’s production and transportation. And because the process creates nitrate, which releases less nitrous oxide during application than ammonia fertilizer does, it has a lower greenhouse gas footprint. On-site production would also give more control to farmers growing food far from manufacturing hubs and in places where instability threatens supply chains.
WHAT’S THE HOLDUP?
Nitrate produced by artificial lightning is still about four times more expensive than industrial ammonia because no one has yet invented a plasma reactor that can fix nitrogen as efficiently as the Haber-Bosch process does.
On balance, the lightning-based process brings down some costs: On-site production means no transport to pay for, and because many plants take up nitrate more efficiently than ammonia, farmers can use less fertilizer for the same crop yield. This also reduces the amount of nitrogen leaching into soil (nitrogen runoff can cause expensive downstream problems, such as algal blooms and fish kills). But technology that is truly cost-competitive will require more research and development.
Several companies are working on this, including PlasmaLeap in Australia and Debye in the UK. A big player in the US recently hit pause: San Francisco–based Nitricity. Once a leader in the space, the company is pivoting to plant-based fertilizer for now, based on surveys of what its clients want. Buy-in from growers could prove a bigger hurdle than perfecting the reactors.
THE UPSHOT
It will take investment—of time, capital, and ideas—before the lightning-based fertilizer process is competitive with the colossus that is Haber-Bosch. But if we get there, we could feed everyone with a scaled-up version of one of Earth’s natural nitrogen fixation processes.
 The Magazine of The Sierra Club
The Magazine of The Sierra Club



