How Does Carbon Capture Work?
We've been putting extra carbon in the air for centuries—here's how to take it out
Capturing carbon dioxide is not a new concept. In the 1920s, some drillers were already venting gas through liquid-filled chambers to separate CO2 from more lucrative methane. The fundamentals of carbon capture—getting carbon molecules near something they'll stick to—haven't changed much since then.
Now the Intergovernmental Panel on Climate Change tells us that in order to maintain a livable climate, we need to not only stop carbon dioxide from entering the atmosphere but also remove billions of tons of the stuff annually by midcentury. Plants, our old allies, can absorb only so much. Far-out ideas like "ocean fertilization" risk trashing entire ecosystems just to store some carbon. Carbon capture can't reverse all our past emissions, but it might help. Here's a look at the leading technologies—promising, hopeful, yet also troublesome.
WAYS TO CAPTURE CARBON
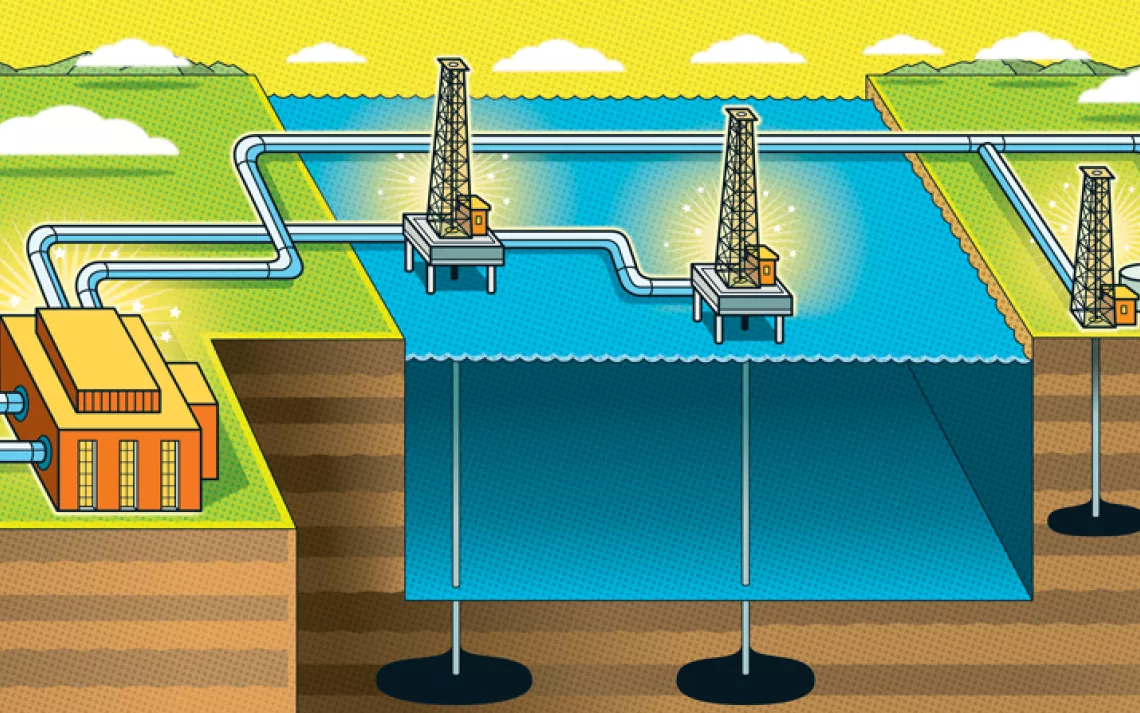
Point-Source Carbon Capture
HOW IT WORKS
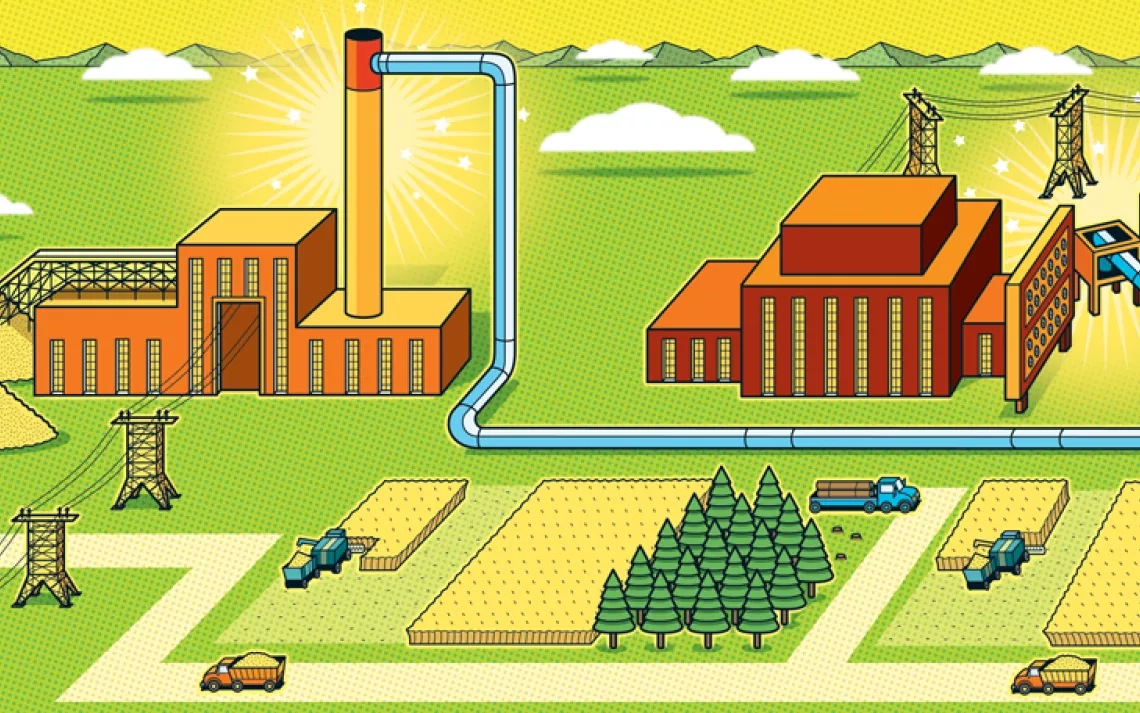
Collecting CO2 from a point source like a smokestack or a gas well is the most efficient form of carbon capture as well as the oldest. Point-source carbon capture costs about $70 per metric ton of carbon—about a fifth of the price of capturing the gas once it's dispersed into the open air.
WHY IT'S PROBLEMATIC
This kind of carbon capture has a long history. For the most part, it's a long history of failure. Point-source carbon capture takes a lot of energy, so the fossil-fueled factories and power plants that use it run less efficiently, use more water, and are more expensive to operate. For that reason, point-source is better suited for industries like steel that are hard to decarbonize by simply switching to renewable energy.
Direct Air Capture (DAC)
HOW IT WORKS
Carbon scrubbers have been making air breathable on space shuttles and submarines for decades—a fan pulls air into a filter that is coated with a chemical that CO2 molecules stick to. Amines, a smelly derivative of ammonia, are a frequent choice, as are soda lime and lithium hydroxide. Heating the filter releases the CO2, which can be stored or sold (though space shuttles and submarines just vent it outside).
WHY IT'S PROBLEMATIC
Snagging CO2 from open air is challenging because the carbon is so diluted—.04 percent versus 15 percent or so in a steel mill smokestack. But if we want a reasonably livable planet, we need to bring that open-air CO2 closer to .035 percent, and that would take hundreds of thousands of DAC plants and a phenomenal amount of energy. Scaling up this technology is not easy. For one thing, it needs renewable energy or the whole setup will generate more CO2 than it can capture.
DAC is also expensive. The Orca plant in Iceland, which is run by the global carbon-capture outfit Climeworks, gets abundant energy from a nearby geothermal plant, but its captured carbon still costs well-heeled buyers $600 to $1,200 per metric ton.
Bioenergy With Carbon Capture and Storage (BECCS)
HOW IT WORKS
With this method, plants (anything from agricultural leftovers like sugarcane stalks to fast-growing crops like poplar trees) do the work of sequestering carbon. The process of burning those plants, capturing the carbon, using the energy, and putting in a new crop is—at least in theory—carbon negative. If you're familiar with ethanol production, you have some idea of how BECCS works. Plenty of ethanol and other bioenergy plants already exist across the United States—the difference is that BECCS plants store or reuse any CO2 released in the production process.
WHY IT'S PROBLEMATIC
The process of vegetation capturing CO2 from the atmosphere is carbon negative. Transporting those plants and refining, capturing, and storing CO2 is not. For BECCS to be carbon negative, all of the above would have to be done with renewable energy.
WHAT TO DO WITH CARBON AFTER IT'S CAPTURED
Use It to Make Something
HOW IT WORKS
Once CO2 is captured, it can be used for a variety of things. It can be pumped into greenhouses to boost growth rates or be transformed into synthetic fuels that can replace diesel, gasoline, and jet fuel, or be used to create chemicals and plastics. It can also be used to make sparkling water (most of the CO2 used to fizz up soda is a fossil fuel byproduct).
WHY IT'S PROBLEMATIC
Carbon Engineering, a Canadian energy start-up, first successfully converted carbon captured from the atmosphere into synthetic fuel in 2017 (it calls this technology "air to fuels"). But in order to pencil out as carbon neutral, that synthetic fuel needs to be created with renewable energy. Also, it would cost several times as much as a gallon of gasoline.
Store It Deep Underground
HOW IT WORKS
After Norway passed a $50-per-ton tax on vented CO2 in the 1990s, the national petroleum company Statoil began to pipe excess CO2 back into the Sleipner undersea gas field. This has been relatively cheap to do, at $17 per ton, and Statoil (now called Equinor) has sequestered about a million tons of CO2 per year since 1996. (That sounds like a lot, but remember that by the second half of this century, we may need to be capturing billions of tons annually.) If done correctly, though, underground storage can be an effective way to sequester CO2 indefinitely.
WHY IT'S PROBLEMATIC
The Sleipner project is just a small part of a much larger "bring gas to the surface of the earth and burn it, thus making climate disruption even worse" operation. That is why undersea carbon sequestration is so popular with oil and gas corporations, which already have the offshore infrastructure and expertise to carry out projects like this.
Without that preexisting infrastructure, and an oil and gas business to help subsidize the cost, piping CO2 underground gets a lot more expensive than $17 per ton. There is also some question as to how much of the CO2 injected into the earth (or below the seafloor) will stay there—a massive fissure was recently found near the Sleipner field, where gas could leak out.
Store It in a Suitable Rock Formation
HOW IT WORKS
In Iceland, Carbfix pumps water filled with CO2 (captured by the Orca project) into basalt rock formations, where it solidifies into carbonate. This currently sequesters about 4,000 tons of CO2 per year—the equivalent of the annual emissions from around 800 cars.
WHY IT'S PROBLEMATIC
If you're trying to sequester carbon for good, this is the gold standard. But it requires a carbon-capture setup to have access to an ideal rock formation.
Use It for Enhanced Oil Recovery
HOW IT WORKS
"Enhanced oil recovery" has been used in the US since the 1970s and is almost always what oil and gas companies are talking about when they tout their carbon-capture projects. A significant percentage of the captured CO2 that is bought and sold worldwide winds up being injected into oil fields to force out more oil. Even cutting-edge carbon projects like SaskPower's Boundary Dam, the first DAC plant to open in North America, sell all the CO2 they capture to local oil and gas outfits.
WHY IT'S PROBLEMATIC
Most CO2 stays trapped underground in this process. But since enhanced oil recovery helps put even more oil and gas on the market, it partially, or even fully, cancels out the benefit of sequestering CO2 in the first place.
THE UPSHOT
For high-tech carbon capture to work, a lot of other things need to work first—and keep on working. Public money needs to be directed toward generating the clean energy necessary to make carbon capture pencil out. Financial incentives need to be reworked so that capturing and storing carbon is more lucrative than releasing it. Corruption and outright carbon fraud need to be watchdogged relentlessly.
The sheer cost of carbon capture serves as an important reminder that investing in cutting emissions right now is a bargain. If funding carbon capture is worth it, so is spending money to not release it in the first place.
This article appeared in the Summer 2022 quarterly edition with the headline "Carbon Captured."
Read why environmental groups are protesting carbon capture pipelines: sc.org/co2-pipelines.
 The Magazine of The Sierra Club
The Magazine of The Sierra Club